why were hydrogen and helium the first elements created
The first "atoms" in the universe of discourse were non atoms at all—they were just nuclei that had not found electrons yet. The simplest nucleus, that of common atomic number 1, is a bare proton with no frills. When the world banged into existence, vigour was rampant. Everything was smashing into everything else. Protons and neutrons often collided, and close to formed larger nuclei, such atomic number 3 that of deuterium (containing a proton and a neutron), likewise as helium nuclei with two protons and two neutrons. Various other arrangements of protons and neutrons also formed, but because the indistinguishability of an atom is determined by its number of protons, all these other conglomerations were au fon just different versions of H, helium and traces of lithium.
Of these three, helium was the first to begin forming "real" atoms. An particle is more than a nucleus—it must also have electrons. Helium nuclei were the first to gather a full purse of electrons en bloc. Why not hydrogen or atomic number 3? Well, helium is the first "noble gas pedal" on the periodic table—the first atom with enough electrons to completely fill the acquirable slots in its electron shell. Thus, if electrons are the currency of chemistry, He is the master pilferer of the oscillating table. In a modern laboratory, IT takes more energy to steal an electron from atomic number 2 than from any other element. And the energy required to remove a second electron is many than twice what information technology takes for the outset. In the early universe, once helium nuclei began to find electrons, they filled the coffers of their electron clouds substantially before the hydrogen nuclei could begin to catch up and in front enough lithium nuclei were even in attendance to accumulate all three of their desired electrons.
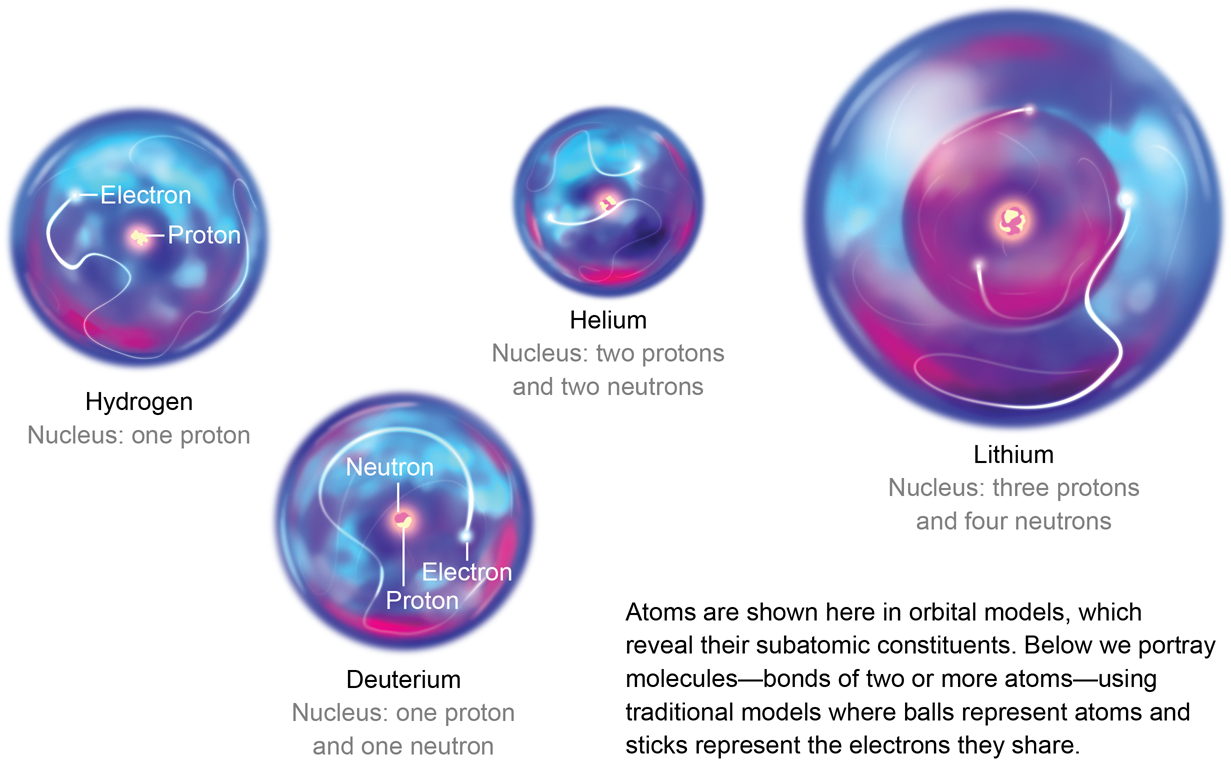
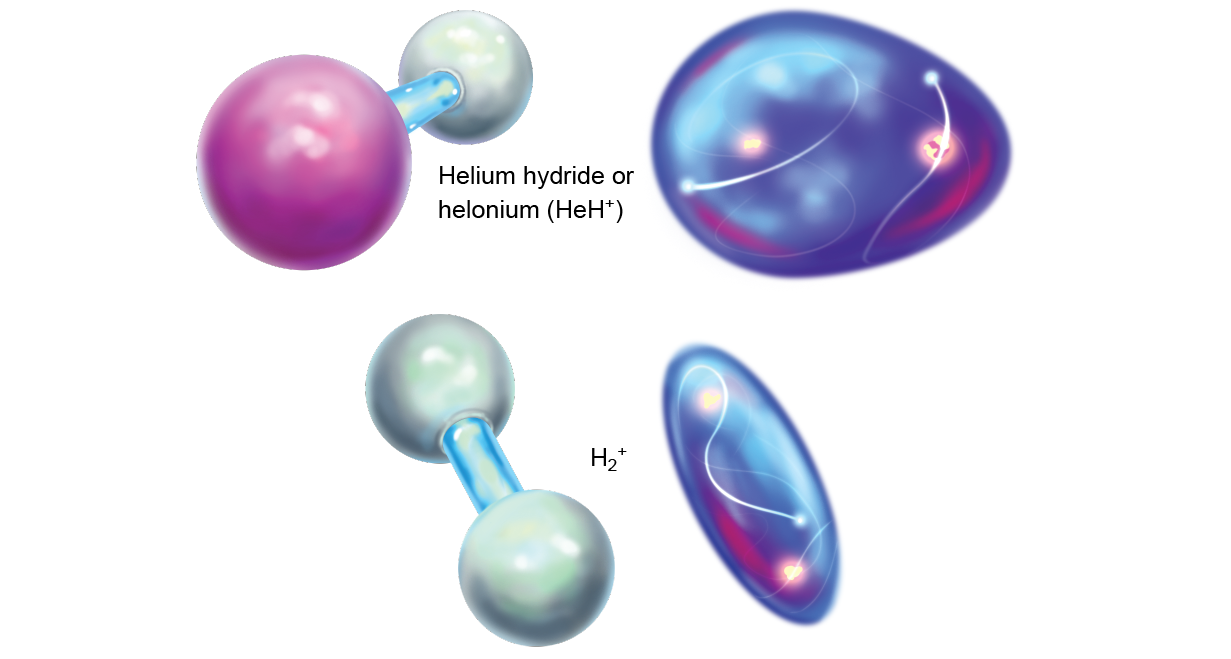
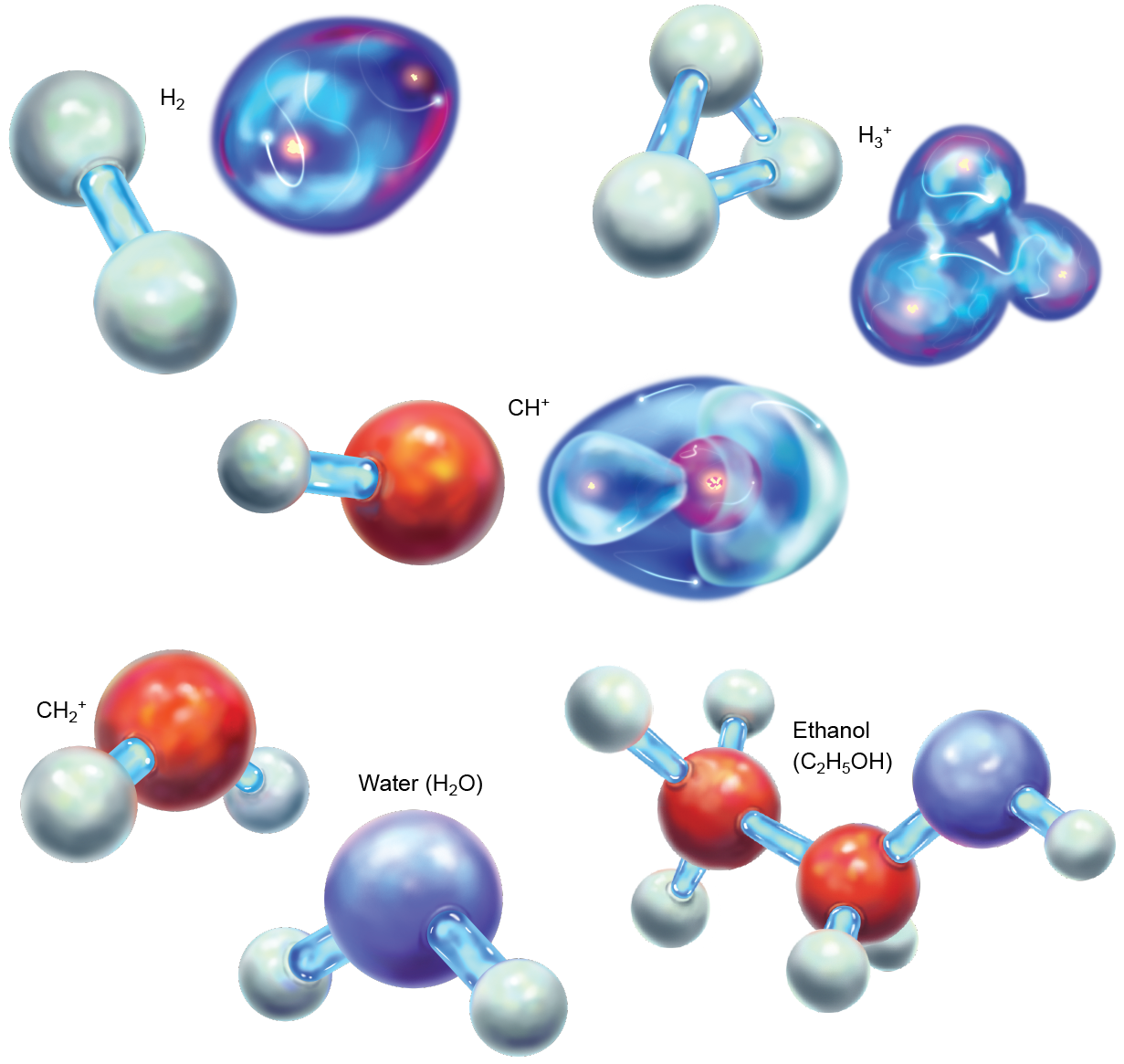
The rest of the matter in the universe at that metre was allay for the most part composed of lone protons, which were starting to feel the effects of being bereft of an negatron. They began slowing down and looking oppositely charged partners to make them electrically neutral. But contracting free electrons for themselves was difficult, and so the protons turned to helium, which already had some. Although helium is loath to share, it unbroken pouring into persistent hydrogen nuclei all the metre. The collisional insistency at length LED a a few helium atoms to share their electrons with protons. Thus, the first off chemical bonds were formed. The new incised of helium and hydrogen was called helium hydride or helonium (HeH+), the rattling first mote (of any continuous abundance) in the universe.
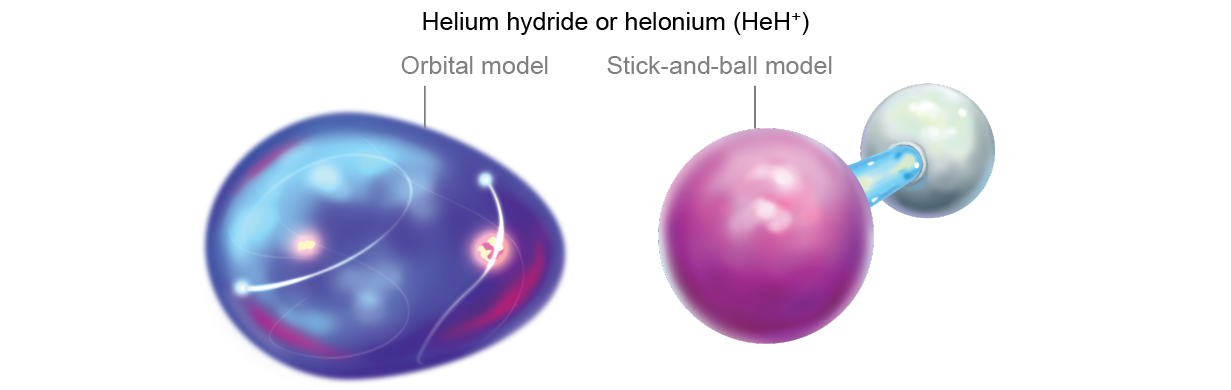
That helium was the first element to bond is surprising because in our current age, we think of helium arsenic the least likely chemical element to relate up with others—the satisfied noble gas with just the right number of electrons. Simply in the early cosmos, He was the only when game in town—the single rely with electrons to loan.
This story has stood on cubic theory-based ground for decades, merely it has long lacked observational certification. HeH+ cannot form on Earth, except in labs, and for decades it went undetected in space. Last year, all the same, astronomers announced that they had observed this molecule for the first metre, lurking in the funeral pyre of a dying star. A 40-year search had paid unsatisfactory, and a new and vital piece was added to our picture of how the early universe took shape.
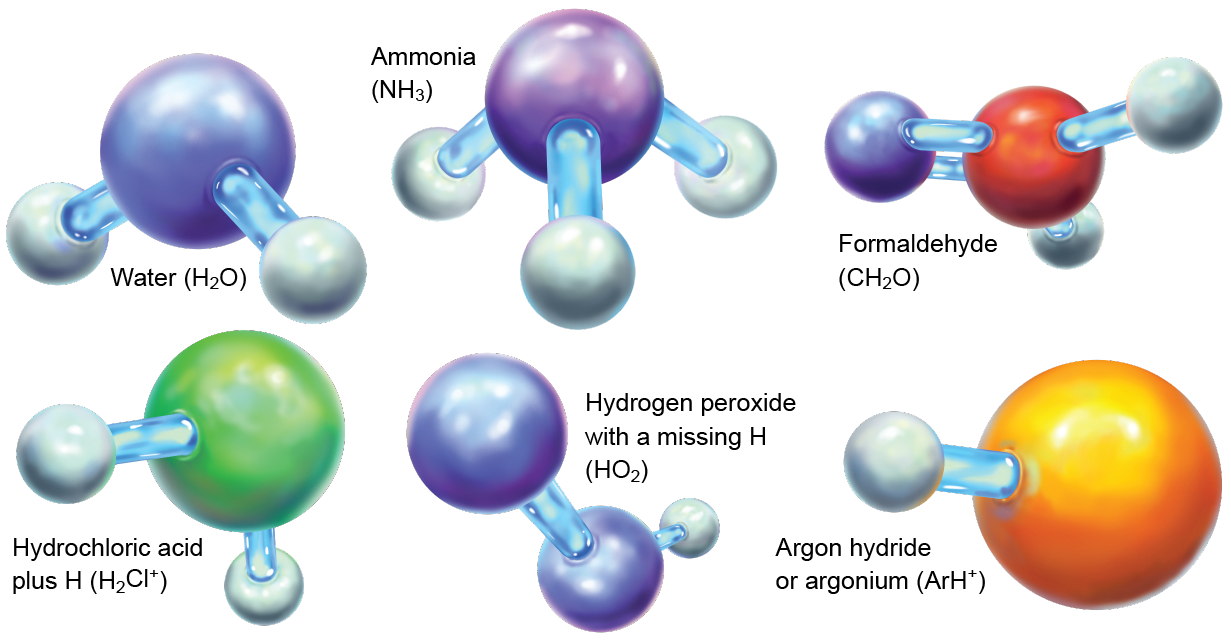
HeH+ now joins the ranks of terrestrial planet molecules; so far scientists have detected more than 200 molecular species in space. This study of chemistry beyond Earth—astrochemistry, as we practitioners like to call up it—is aimed at clarifying what molecules are present in space, how they form, and what their organic evolution means for observational and theoretical astrophysics. Many of the known astromolecules, including water, ammonia and formaldehyde, are common here on Earth. Others are mundanely flakey, such as hydrochloric acid with an extra proton and hydrogen peroxide with one of its hydrogen atoms amputated. Provocative molecules, systems with unmatched electrons and strange arrangements of atoms in other than common molecules get likewise been observed. We have plane seen molecules containing the so-called inert noble gases, such atomic number 3 ArH+ (a jazz group of argon and hydrogen) and the newly registered HeH+.
Virtually disciplines of chemistry are focused on devising the worldly concern safer, more efficient or more enjoyable for humans. Astrochemistry, withal, looks at the nearly key properties of molecules. It helps to define what soldering really is, how protracted molecules can remain intact and why definite chemical species are more than inferior than others. By studying chemistry in environments and then very estrange compared with Earth—with temperatures, pressures and available ingredients quite different from what we are ill-used to—we can find molecules that dispute our usual notions of how atoms interact and that bring us to a deeper chemical substance understanding. Ultimately we trust to learn how chemistry LED to the ingredients that ended up in the planets in our star system and eventually enabled life.
Where Was HeH+?
In a University of California, Berkeley, research laboratory in 1925, T. R. Hogness (WHO advanced worked on the Manhattan Project) and teaching fellow E. G. Lunn found that mixing helium and hydrogen gas in the presence of an electric arc within a vacuum chamber could create different ions with antithetic masses. Measuring the mass-to-charge ratio of molecules is the fortemente of the chemical discipline called mass spectroscopy; the early implementation of this now common chemical proficiency showed that this variety produced a transient mass-to-charge ratio of 5. That could only be HeH+. Merely keeping this argonon molecule around long sufficiency to study it proved exceptionally difficult, even in Hogness and Lunn's controlled lab.
In the early cosmos, it would have been even out more coseismal because HeH+ is likely to let go of of its proton on even the slightest contact with another particle. In this relationship, atomic number 2 gives two electrons, whereas hydrogen gives none. Such uneven soldering (called dative case bonding) is weaker than traditional covalent bonds, in which some atoms bestow more evenly.
In 1978 John H. Black, then at the University of Minnesota, was the first to argue that HeH+ could still represent present in space. Black suggested that a good place to look back was planetary nebulae, the puffed-out and highly energized matter created in a star's death throes. In these clouds, a thin layer of ionized helium atoms is typically found in the presence of neutral hydrogen atoms; helium's strong need for electrons could drive it to borrow same from hydrogen, creating a bond. Consequently, since the late 1970s astronomers and their pill pusher collaborators have been looking for HeH+ in myriad places, from the edge of the universe to supermassive stars. Yet for decades these searches found nothing, leading some to uncertainty the validity of HeH+'s role in jump-starting chemistry. Did helium really enslaved with H+? It seemed like information technology must have; in that respect was nothing else to bond with spinal column so. Only if that were the pillowcase, then where was HeH+?
Unit Fingerprints
While astrochemists were looking for HeH+ and coming skyward meaningless, researchers found many unusual molecules they were not expecting. They could not symmetric identify some of them.
It began in 1919, when Mary Lea Heger was using the Clobber Observatory on summit of Mount Hamilton in Santa Clara County, California, to notic the behavior of a match of orbiting binary stars, a twin organization akin to the suns of Tatooine. What she sawing machine was surprising.
Each molecule has its own system of atoms and electrons and thus absorbs brightness in a unparalleled right smart. These "absorption features" give all molecule its ain set of fingerprints, seen when astronomers separate incoming light into its grammatical constituent wavelengths—a process known as spectroscopy. A Heger's multiple stars orbited their central point of gravitation, the spectral features in each genius's atmospheric state also shifted in wavelength (the Doppler effect).
But Heger as wel found some ghostlike fingerprints that were regular still as the stars moved around. She then looked at another binary sensation system and saw the same pattern. Come after-up lic showed that these rigid features also showed up when telescopes were aimed toward single stars. The imprints must have been orgasm from molecules not more or less stars but in the immense, cold regions between them. The craziest part was that basically the same fingerprints were attending for every last observed stars and justified for other galaxies. The signatures, dubbed circularize interstellar bands (DIBs), were everywhere. Scientists scoured the documented spectral features of molecules on Earth, recently synthesized ones from labs and those observed in quad through radio-telescopic fingerprinting. Nothing matched the DIBs—they were something novel.
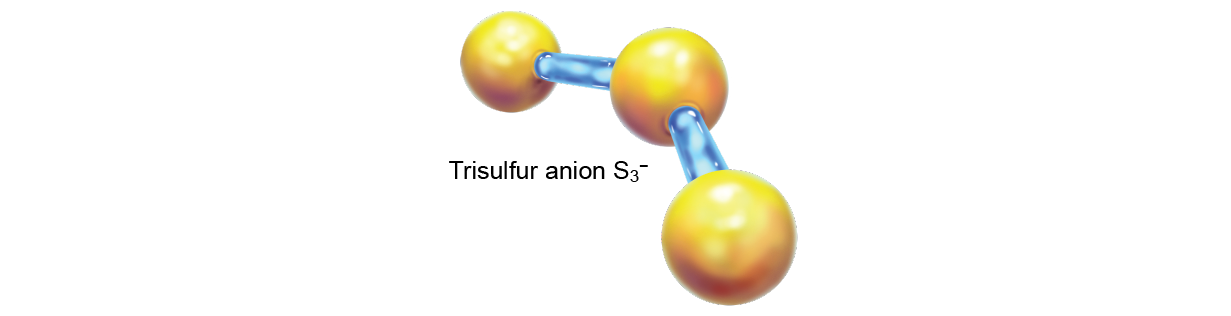
The late Harvard University professor William Klemperer, unity of the foremost pioneers of astrochemistry, once suggested that the DIB signatures might belong to the trisulfur anion, S3 –. When this proved untrue, he was so dejected that he wrote, "In that respect is no better way to lose a scientific reputation than to speculate on the carrier[s] of the diffuse [interstellar] bands." Hypotheses atomic number 3 to the provenance of the DIBs circulated through the decades, but none stuck—it was called the longest-permanent problem in spectroscopy.
One of the most challenging hypotheses proposed polycyclic aromatic hydrocarbons (PAHs) equally a DIB defendant. PAHs—hexagons of carbon atoms arranged stunned in sheets—are the stellar component of crock, asphalt and graphite. They are unlikely to react with other molecules simply do tend to stick with them. For astrochemists, the trouble with PAHs is that their many varieties are soh similar to one another that their spectroscopical fingerprints, operating theater spectra, run together. It is like trying to spot the person brushstrokes of Vincent van Gogh's Starry Night instead of seeing the full painting—the many parts are subsumed by the full-page. But the DIBs seemed to behave in a similar fashion. Could PAHs explicate the DIBs?
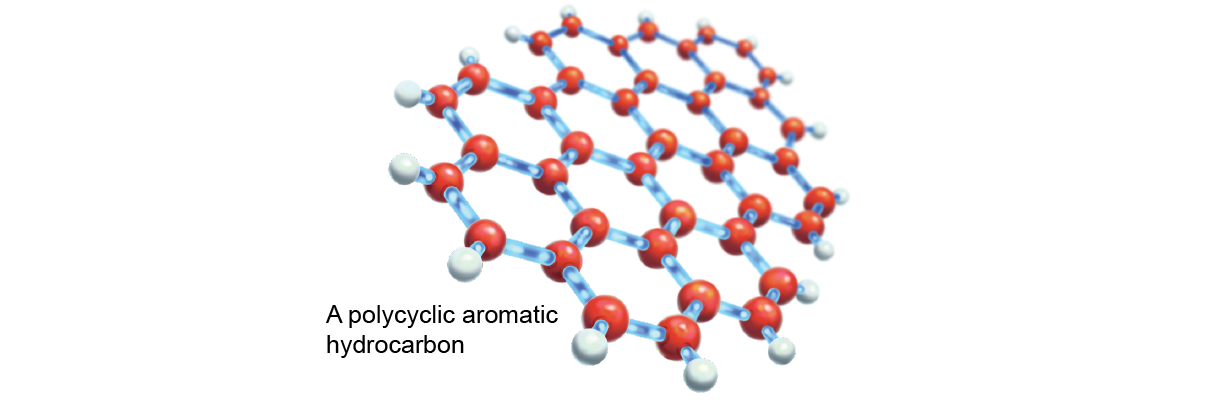
Such ideas cause bounced around in astrochemistry circles since the 1970s, but one experiment forever varied how we think about carbon. Harry Kroto, who died in 2016, was at the University of Sussex in England in the 1980s and worked along a team to detect new molecules in space. He heard about an experiment by Robert F. Curl and Richard E. Smalley, both chemists at Rice University at the fourth dimension, in which they had ablated an aluminum surface and found all kinds of new aluminum molecular clusters. When they substituted graphite (a questionable grand-PAH) for aluminum, a most bizarre molecule appeared: C60, 60 carbon atoms arranged like a association football orchis. In 1996 Kroto, Curl and Smalley were awarded the Nobel Prime in Chemical science for their roles in discovering the molecule, called buckyball, or just fullerene (also titled a buckyball). Kroto was convinced that buckyballs were present tense in space and were likely to be the source of more or less DIB fingerprints. Only few the great unwashe believed him, though, and atomic number 2 and his colleagues moved happening. Nevertheless in 2010, a fourth part of a century afterward their initial discovery in the laboratory, C60 and its cousin C70 were observed in the infrared in planetary nebula Tc1 in the constellation Cygnus. Whether these molecules were, in fact, related to the visible-wavelength DIBs was calm down indecisive. Theoretical work out recommended so, but scientists lacked substantiative data-based data.
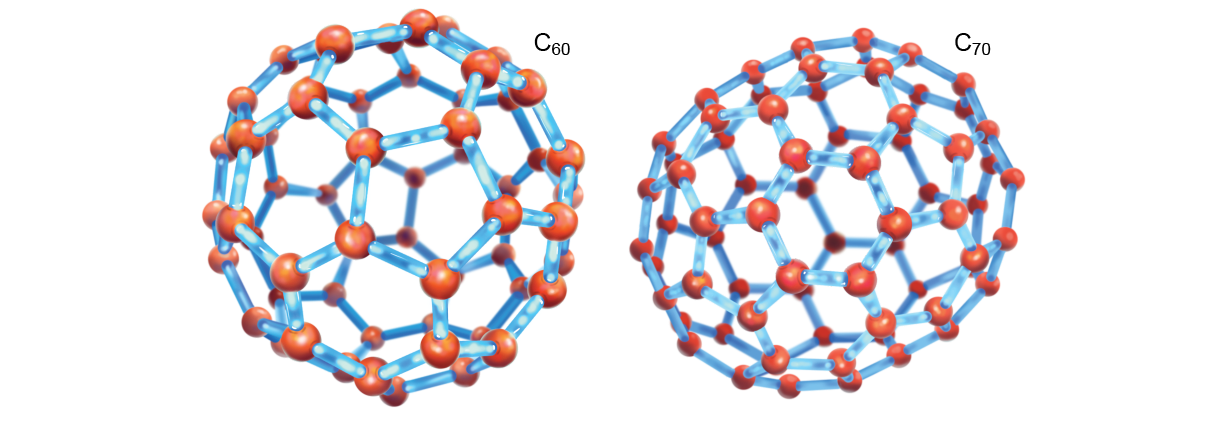
In 2015 the cation form of fullerene, C60 +, was finally trapped in the science laborator, and scientists were able to once and for all measure its near-infrared spectrum. One, then two lines from this speck matched known DIB wavelengths. Later, researchers showed that these fingerprints matched four or five DIBs. Then, in 2019, an international team led by Martin A. Cordiner of NASA's Goddard Space Flight Center used the Hubble Space Telescope to examine the DIB wavelengths seen in the direction of 11 by and large red (older, bigger) stars and set up that they matched the experimental data for C60 +, confirming at last that this atom's fingerprints are responsible for some of the DIBs.
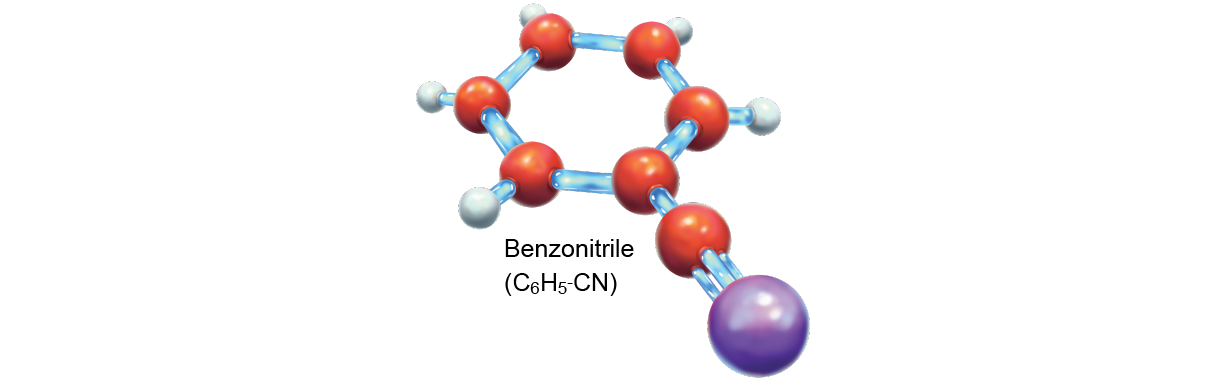
This discovery indicates that at least one type of speck once and for all leaves its fingerprints all over celestial body space. Buckyballs are believed to germinate from PAHs, and their presence in space implies that their parent molecules must as wel be out there. Yet it was non until 2018 that researchers observed the fingerprints of a PAH-family molecule in place. The compound they saw, benzonitrile (C6H5-CN), is a rare aromatic hydrocarbon that is more easily heard than its relatives. And regular more recently, scientists observed double-ring cyanonaphthalene molecules, revealing that larger PAHs are present as well.
Discovery
Despite these breakthroughs, for a years HeH+ remained elusive.
The first molecules would have dissipated fairly quickly aft the earliest epochs. Arsenic the universe matured, expanded and cooled, the leftover hydrogen nuclei began to gather electrons of their own. At that point these now neutral atomic number 1 atoms presumptively matte the positive charge on the HeH+ molecules. When the atoms and molecules collided, the comparatively weak He-H dative case attachment broke, and a a good deal stronger valence bond between two hydrogens formed to make up H2 +. Afterward that, the helium atoms were largely left alone.
It might appear, so, that the brief existence of HeH+ was inconsequential, but that is far from the event. Models of potential chemical reactions in this period indicate that without HeH+ formation, H2 +, and then neutral H2, would have close so much many slowly. Once H2 had been made, though, the entire tree of chemistry unfolded. Next came H3 +, which begot CH+, which begot CH2 + and a cascade of other molecules. Eventually this mountain range led to water, grain alcohol and larger species. These processes are complete the product of the unbalanced bonding in HeH+; without this initial relation, the universe would be a different place.
Still, by 2013 astrochemists were getting foiled that HeH+ was nowhere to glucinium found. But that year a auspicious sign came when researchers disclosed the related noble gas molecule ArH+ in the Phthirius pubis Nebula supernova leftover. Scientists focused the search for HeH+ in confusable, superenergized environments. The large job, though, was that the spectra of HeH+ fell in the same neighborhood as fingerprints of the very world-class molecule ever ascertained in space, the CH radical. No more telescopes had the business leader to separate these signatures.
Then along came the Stratospheric Observatory for Infrared Astronomy (SOFIA), a repurposed 747 jumbo jet with a hole cut in its side so an infrared telescope force out look kayoed. In May 2016 an International team up used SOFIA, a joint project of NASA and the German Aerospace Center, for three nights of observations. The SOFIA scope has the resolution requirement to tell apart HeH+'s unique rotational-frequency fingerprint at 2,010.184 gigahertz. Thither, in the haystack of far-invisible information within another burned-out cinder of an exploded star in the planetary nebula NGC 7027, part of the constellation Cygnus, was the fingermark that had gone missing for bye. This hellish place, with its high-topped temperatures and energies, was not unlike the early universe. Happening April 17, 2019, a team up led by Rollo Güsten of the Max Planck Found for Radio Uranology in Bonn, Germany, published a report in Nature heralding the discovery of HeH+.
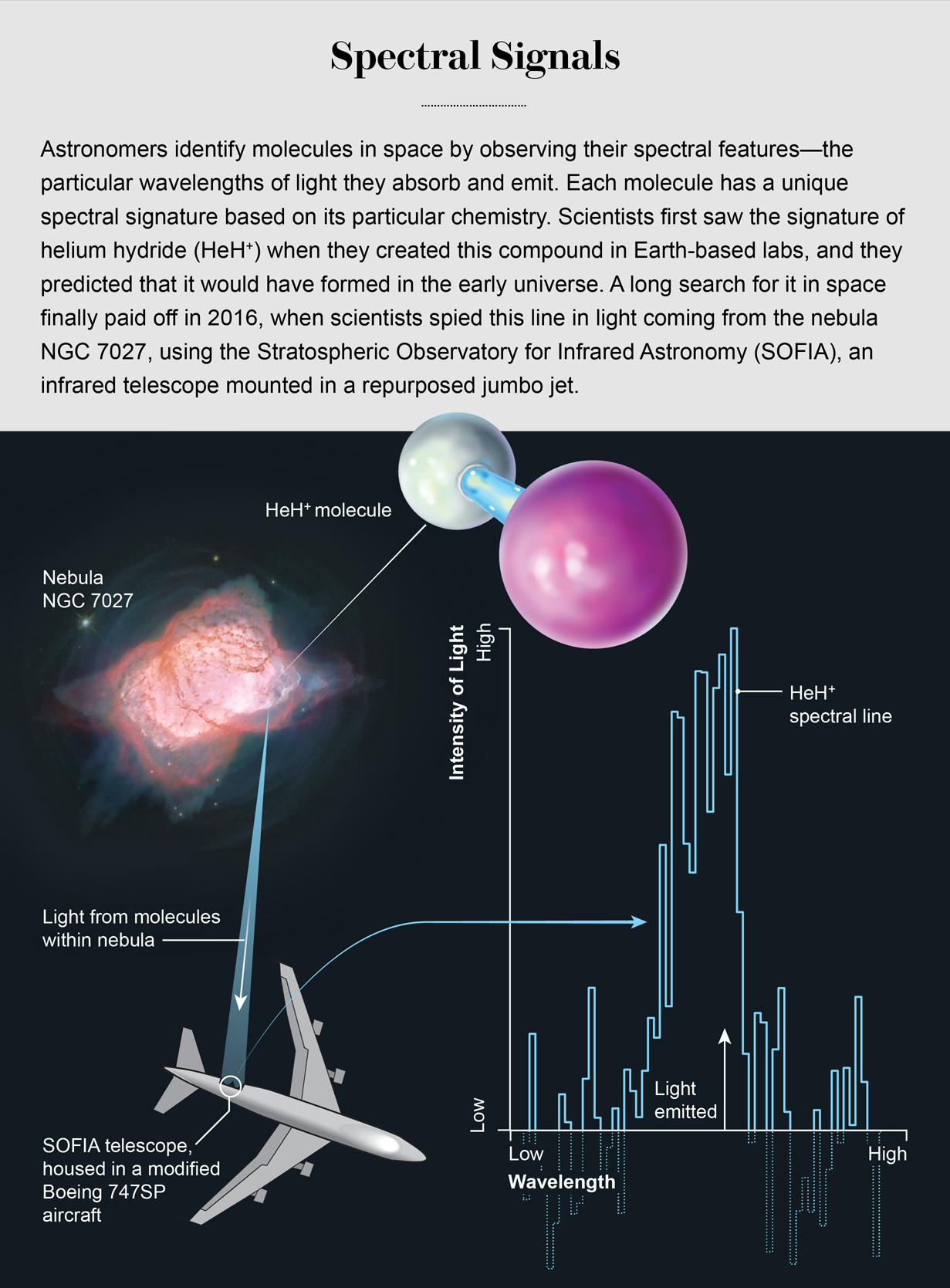
Given, this sighting is not of primordial HeH+. We believe that the molecules Güsten and his colleagues observed were created much more lately. Nevertheless, the determination helps to constrain our knowledge of this compound. Scientists tush now intent better models of the universe as it existed when HeH+ was the only molecule in townspeople. The discovery might also give us clues about where other this chemical may be lurking in blank nowadays, directing United States toward other international nebulae or even other regions of space that are so far away they correspond to earlier epochs of time, going back to the edge of the universe.
Harder Questions
This is an exciting time in astrochemistry. Tercet grand questions have been conclusively answered in quick succession. Scientists have observed the first molecule to frame in the cosmos and identified the first fingerprints belonging to the mysterious DIBs, and they are finally elucidating PAHs from the blackness of space.
To boot, lab simulations of interstellar conditions are showing how amino acids and nucleobases might have formed. Space telescopes such as SOFIA and Hubble, as advisable atomic number 3 the future James I Sidney Webb Space Telescope, promise to provide unprecedented spectral characterization of star objects where young, less common building block fingerprints may be seen.
Now that we are determination answers to these far-famed problems, other quandaries are popping up. Eventually astrochemists hope to fishing gear harder questions, such as "What are the rest of the DIBs?," "What are the building block origins of life?" and "What chemical mix is necessary for the formation of rocky planets rather than brag giants?" It was the sharing of electrons that created observable matter in the creation. When we have a deeper comprehension of these chemical processes, we canful gain a better-grain understanding of astrophysics and the overall history of our universe.
why were hydrogen and helium the first elements created
Source: https://www.scientificamerican.com/article/the-first-molecule-in-the-universe/
Posting Komentar untuk "why were hydrogen and helium the first elements created"